Any substance that has mass and volume is considered matter since it occupies space.
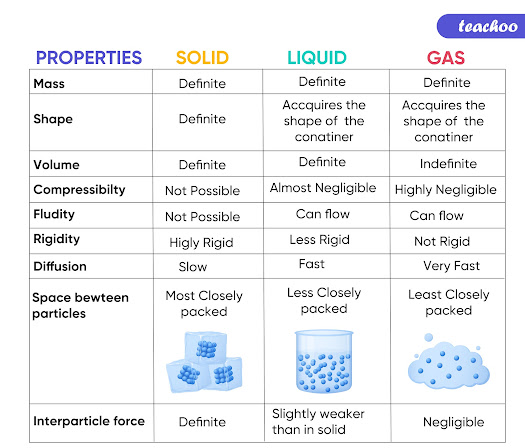
Matter exists in three different states: solid, liquid, and gas. By examining the configuration of their particles, it is possible to understand why they have various qualities. Theoretically, at this temperature, particles move slowly and have the least amount of energy.
Solids: Solids have fixed shapes along with fixed volumes. Solid particles are arranged closely in a repeating and regular pattern. Solid particles are held or composed in place by attractive forces. Particles in solids do not move, instead they vibrate in place which makes solids the state with the least kinetic energy.
Liquids: Liquids have no fixed shape as they take the shape of their container, but they do have fixed volumes. Liquid particles are arranged in a close non-regular patters. The liquid particles roam around freely around each other held by attractive forces to keep them together. The kinetic energy in liquids is greater than the kinetic energy in solids.
Gases: Gases have no fixed shapes nor volumes. Gas particles flow freely from each other due to the insignificance of the attractive forces in them. Gas particles possess the greatest kinetic energy out of all three states.


Really great explanation! thank you!
ReplyDelete